Gold, a precious metal highly valued for its beauty, rarity, and unique properties, has been mined and refined for centuries. Among the various types of gold ores found in nature, sulfide gold ore plays a significant role in global gold production.
In these ores, gold exists in the form of metal sulfides, primarily including pyrite (FeS₂), arsenopyrite (FeAsS), and, more importantly, gold-silver tellurides and natural gold mixed with sulfides. Understanding the characteristics of sulfide gold ore and the processes for extracting gold from it is crucial for the mining industry.
Introduction
Sulfide gold ore refers to a type of geological deposit in which gold is locked within metal sulfide minerals. These ores typically have complex compositions, containing not only gold but also silver, copper, lead, zinc, and other metals. As a result, their processing is more complicated than that of free gold ores (those that release gold more easily during processing). Gold in sulfide ores is usually dispersed in fine particles within the sulfide matrix, requiring specialized extraction techniques.
Characteristics of Sulfide Gold Ore:
Gold Occurrence
Gold is typically dispersed in fine particles within sulfide minerals, often encapsulated by these minerals. This state makes it difficult to extract gold, and traditional extraction methods often fail to effectively recover the encapsulated gold.
Complexity of the Ore
In addition to gold and sulfide minerals, sulfide gold ore often contains other impurity minerals, increasing the complexity of ore processing. These impurities not only affect the efficiency of gold extraction but may also interfere with subsequent processing steps.
Low Leaching Efficiency
Due to the encapsulation of gold by sulfide minerals, the leaching rate of gold is often low when directly using cyanide extraction. This characteristic necessitates additional pretreatment steps in traditional gold extraction methods.
FORUI will share with you a commonly used mineral processing technique for difficult-to-process sulfide gold ore:
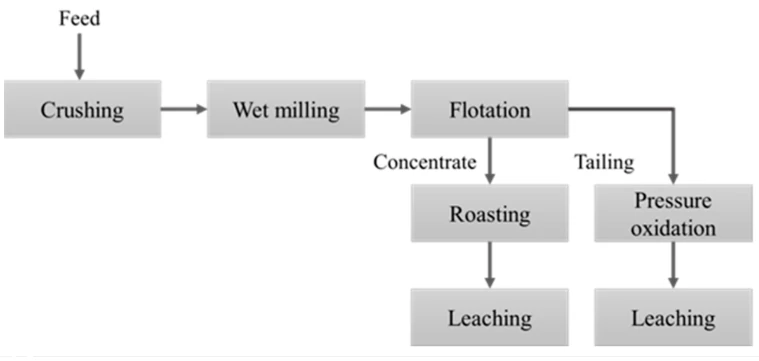
Flotation-Roasting-Cyanidation
Flotation
Objective: To concentrate the sulfide minerals containing gold.
Process: The ground ore is mixed with water, reagents, and air. The gold-containing sulfide mineral particles become hydrophobic, attach to air bubbles, and form a froth that is then skimmed off. This process can effectively increase the gold grade and reduce the amount of material for subsequent processing.
Roasting
Objective: To oxidize sulfide minerals and release the encapsulated gold.
Process: The sulfide concentrate is heated in air at high temperatures, converting sulfides into oxides while releasing sulfur dioxide gas. For example, the reaction equation for pyrite is:
[ 4FeS₂ + 11O₂ \rightarrow 2Fe₂O₃ + 8SO₂ ]
Environmental Issues: The sulfur dioxide produced during roasting can cause air pollution, necessitating appropriate environmental protection measures.
Cyanidation
Objective: To dissolve gold in a sodium cyanide solution.
Process: The roasted ore is leached with sodium cyanide solution, forming a soluble gold-sodium cyanide complex. The reaction equation is:
[ 4Au + 8NaCN + O₂ + 2H₂O \rightarrow 4NaAu(CN)₂ + 4NaOH ]
Gold Recovery: Gold is recovered from the solution through methods such as zinc displacement or activated carbon adsorption.

More individual consulting
Get in touch and talk to our experts.
We’re happy to help: contact us here.
Advantages and Disadvantages of the Processing Methods
Advantages
Wide Applicability: This process is suitable for various types of sulfide gold ores.
High Efficiency: Flotation concentration can significantly enhance the gold grade, reducing the volume of material for subsequent processing.
Wide Applicability: This process is suitable for various types of sulfide gold ores.
Disadvantages and Environmental Issues
Environmental Impact: The sulfur dioxide gas generated during roasting and potential water pollution from cyanidation are critical issues that need to be addressed during processing.
High Energy Consumption: Roasting requires high temperatures, resulting in significant energy consumption.
Modern Alternative Methods and Improvements
To address the environmental and economic issues associated with traditional gold ore processing methods, researchers have developed several new approaches:
Bio-Oxidation: Utilizing microorganisms to oxidize sulfide minerals can effectively reduce pollutant emissions from the roasting process.
Thiosulfate Leaching: Using sodium thiosulfate solution to dissolve gold can avoid the use of cyanides, thereby reducing environmental hazards.
Modern Alternative Methods and Improvements
The extraction of gold from sulfide gold ores presents unique challenges due to the encapsulation of gold in sulfide minerals. Various pretreatment processes, such as roasting, pressure oxidation, and bio-oxidation, can effectively release the encapsulated gold, paving the way for subsequent cyanidation or other extraction methods. Although these processes may be more complex and costly, ongoing technological advancements are significantly improving the efficiency and environmental sustainability of these methods.